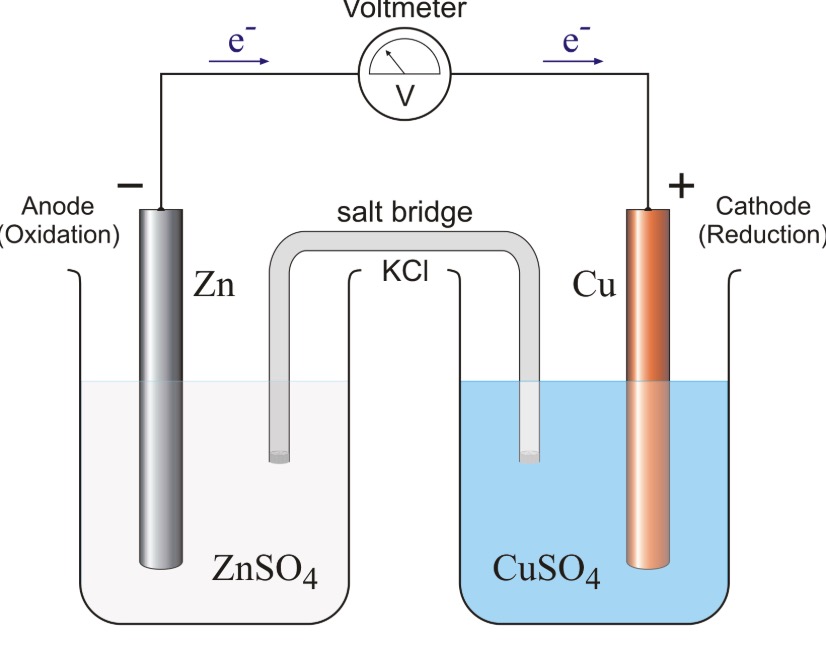
Distinguish Between Galvanic Cell And Electrolytic Cell . Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web there are two types of electrochemical cells: Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web both a galvanic cell and an electrolytic cell have electrical current flowing.
from classnotes.org.in
Web there are two types of electrochemical cells: Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to.
Electrochemical Cells Chemistry, Class 12, Electro Chemistry
Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web there are two types of electrochemical cells: Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to.
From ar.inspiredpencil.com
Galvanic Cell Labeled Distinguish Between Galvanic Cell And Electrolytic Cell Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis. Distinguish Between Galvanic Cell And Electrolytic Cell.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co Distinguish Between Galvanic Cell And Electrolytic Cell Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Galvanic cells. Distinguish Between Galvanic Cell And Electrolytic Cell.
From www.goconqr.com
Electrochemistry Mind Map Distinguish Between Galvanic Cell And Electrolytic Cell Web there are two types of electrochemical cells: Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web both a galvanic cell and an electrolytic cell have electrical current flowing. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web a galvanic cell. Distinguish Between Galvanic Cell And Electrolytic Cell.
From www.pinterest.co.uk
What are the differences between Galvanic cell and Electrolytic cell Distinguish Between Galvanic Cell And Electrolytic Cell Web there are two types of electrochemical cells: Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web a galvanic. Distinguish Between Galvanic Cell And Electrolytic Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Distinguish Between Galvanic Cell And Electrolytic Cell Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web both a galvanic cell and an electrolytic cell. Distinguish Between Galvanic Cell And Electrolytic Cell.
From llivetolearn.blogspot.com
Live to Learn, Learn to Live Electrochemical cells, Difference between Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web there are two types of electrochemical cells: Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web a galvanic (voltaic). Distinguish Between Galvanic Cell And Electrolytic Cell.
From allthedifferences.com
What Is The Difference Between Electrolytic Cells And Galvanic Cells Distinguish Between Galvanic Cell And Electrolytic Cell Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web there are two types of electrochemical cells: Web. Distinguish Between Galvanic Cell And Electrolytic Cell.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube Distinguish Between Galvanic Cell And Electrolytic Cell Web there are two types of electrochemical cells: Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis. Distinguish Between Galvanic Cell And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Distinguish Between Galvanic Cell And Electrolytic Cell Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web there are two types of electrochemical cells: Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web this article. Distinguish Between Galvanic Cell And Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web a galvanic cell is. Distinguish Between Galvanic Cell And Electrolytic Cell.
From employees.csbsju.edu
CHEM 123 GENERAL CHEMISTRY 1 Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web a galvanic (voltaic) cell uses the energy released during a. Distinguish Between Galvanic Cell And Electrolytic Cell.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web both a galvanic cell and an electrolytic cell have electrical current flowing. Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis. Distinguish Between Galvanic Cell And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web there are two types of. Distinguish Between Galvanic Cell And Electrolytic Cell.
From www.clutchprep.com
Electrochemical Cells Analytical Chemistry Video Clutch Prep Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Galvanic cells utilize a spontaneous flow of electrons to. Distinguish Between Galvanic Cell And Electrolytic Cell.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Distinguish Between Galvanic Cell And Electrolytic Cell Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web there are two types of electrochemical cells: Web. Distinguish Between Galvanic Cell And Electrolytic Cell.
From www.youtube.com
The differences between the voltaic cell and the electrolytic cell Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web there are two types of electrochemical cells: Web both a galvanic cell and an electrolytic cell have electrical current flowing. Web a galvanic cell uses. Distinguish Between Galvanic Cell And Electrolytic Cell.
From differguide.com
Difference Between Galvanic And Electrolytic Cell Distinguish Between Galvanic Cell And Electrolytic Cell Galvanic cells utilize a spontaneous flow of electrons to convert chemical energy to electrical energy. Web there are two types of electrochemical cells: Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web. Distinguish Between Galvanic Cell And Electrolytic Cell.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Distinguish Between Galvanic Cell And Electrolytic Cell Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web a galvanic cell uses spontaneous redox reaction to generate electrical current and an electrolytic cell uses electrical current to. Web a. Distinguish Between Galvanic Cell And Electrolytic Cell.